Recent breakthroughs in optical-based imaging in nonhuman primates promise to fundamentally advance our understanding of brain function and accelerate the development of next-generation brain-computer interfaces.
- Two new studies demonstrate complementary approaches for imaging the activity patterns of large populations of neurons in nonhuman primates (NHPs).
- Bollimunta*, Santacruz* & colleagues used a head-mounted one-photon miniature microscope to image the activity of neurons deep in the motor cortex of a behaving macaque, and demonstrated the ability to perform offline decoding of the animal’s motor behavior using the same neurons tracked over weeks.
- Trautmann*, O’Shea*, Sun* & colleagues used two-photon microscopy to image the activity of neurons, also in the motor cortex, and show that it is possible to access deep cortical neurons by imaging their apical dendrites near the surface, allowing them to perform online, closed-loop decoding of the behaving macaque’s motor behavior sufficient to drive an optical brain-computer interface (BCI).
- Together, these studies pave the way for new experiments to understand how populations of neurons underlie behavior, and how new BCI technologies can treat neurological injury and disease.
Recent breakthroughs in neuroscience, engineering and medicine have set the stage for entirely new ways to treat brain disorders and restore physical and cognitive abilities. Implantable medical devices have the potential to treat paralysis, restore lost senses, remedy depression, and to assist faltering memory—challenges for which drugs may be ill-suited. Brain-computer interface (BCI) technologies offer a direct avenue to remediate nervous system injury and disease with precision and efficacy. Advances in basic neuroscience and engineering are both required for medical BCIs to realize widespread clinical impact.
Basic neuroscience has made numerous foundational breakthroughs in recent years, built on major advances in our ability to record from and interact with individual neurons configured into circuits (Vázquez-Guardado et al. 2020; Macknik et al. 2019). Optical technologies, such as calcium imaging and optogenetics, make it possible to read information from and write information to neural circuits with single-cell resolution, and allow experimenters to use genetic tools (e.g., viral vectors) to target specific cell types or anatomical projections. Calcium imaging, which relies on genetically encoded fluorescent sensors (e.g., GCaMP) to track intracellular calcium levels as a proxy for neuronal spiking activity, now enables recording large populations of neurons (tens to hundreds of thousands) across multiple brain areas in rodents and has already yielded important new insights into the neural circuit mechanisms underlying essential brain functions.
Prior to the advent of calcium imaging, extracellular electrical recordings were the only available method for recording large populations of neurons. While today’s most advanced multi-electrode array technologies have many merits, including millisecond-scale temporal resolution, they also suffer from several key limitations.
Chief among these limitations is that they are typically blind to the neuronal subtype identity of recorded neurons, whereas optical methods, as already mentioned, allow for recordings from specific anatomically and genetically-defined neuronal subtypes. Another important limitation of electrophysiological recordings is their relatively sparse spatial sampling density, requiring one electrical contact for every sampling location. Even recent high-density electrodes, such as Neuropixels probes, sparsely sample a small volume of tissue (Leber et al. 2019; but see Jun et al. 2017; Trautmann et al. 2019; Steinmetz et al. 2021). In contrast, optical methods allow for dense sampling of all neurons within a recording volume at single-cell resolution. Finally, electrophysiological approaches are unable to reliably track the same neurons beyond a single recording session, whereas optical methods make it relatively straightforward to do so across several weeks to months.
Researchers have used the powerful capabilities of calcium imaging techniques to demonstrate the enormous potential for all-optical BCI in rodents (Clancy et al. 2014). Extending optical imaging techniques to nonhuman primates (NHPs) presents the possibility of fundamentally transforming our understanding of the primate brain and informing next-generation clinically viable BCIs for humans (O’Shea et al. 2017). Rhesus macaque monkeys (Macaca mulatta) are a particularly important model species in neuroscience and translational research since their brain structure and function, as well as complex cognitive and behavioral abilities, are highly similar to those of humans. Macaques exhibit a high degree of cognitive flexibility and are capable of learning a rich repertoire of sophisticated, precision behaviors. Investigations using macaques have served a vital role in developing clinically-viable BCIs, by exploring decoding algorithms and system designs and by advancing our basic scientific understanding of the motor system (Nuyujukian et al. 2017). Recently, researchers have been able to use calcium imaging techniques in macaques to study the visual cortex (Seidemann et al. 2016; Ju et al. 2018; Li et al. 2017), towards the rear of the brain. These successes in the primate visual system pave the way for using arm-movement related calcium signals from neurons in the motor cortex to drive an optical BCI.
Until now, BCI studies in NHPs have used intracortical multi-electrode arrays, which are also used in BCI clinical trials (i.e., Utah arrays), to facilitate translation of scientific and technical achievements from NHP pre-clinical research into advanced BCI designs in clinical trials. For some aspects of BCI implementation and translation, such as studying biocompatibility, stability, and longevity, using the same implantable sensor is of central importance. For other aspects of BCI experimentation, however, the central goal is the scientific study of how neural populations perform computations and how relevant signals of interest can be optimally decoded. Such studies aim to obtain fundamental understanding that can inform the design of future high-performance and highly-robust BCI systems (Gilja et al. 2012; Nuyujukian et al. 2014, 2015; Kao et al. 2016; Nuyujukian et al. 2017; Gilja et al. 2015; Pandarinath et al. 2017; Nuyujukian et al. 2018; Stavisky et al. 2019; Willett et al. 2021; K. Shenoy and Yu 2021). Optical-based BCI in NHPs is therefore well poised to play a critical role in advancing these goals.
Conventional widefield and two-photon calcium imaging, commonly used in rodents and other small animal models, is limited to imaging the very upper layers of cortex, due to the scattering of photons in the brain tissue. Two recent studies have now demonstrated complementary approaches for imaging populations of neurons beyond the conventional limits of calcium imaging methods. These approaches also address the limitations of electrophysiology, allowing for genetic targeting and dense imaging of large populations of neurons deep in the brain, and are the first studies to use optical imaging to record from macaque motor cortex.
In the first, a collaboration of researchers, led by Anil Bollimunta of Inscopix Inc and Samantha Santacruz of UT Austin, developed custom implant hardware and methods for imaging neurons deep in the cortex using microendoscopic probes and head-mounted miniature microscopes (Figure 1A-D; Bollimunta, Santacruz et al. 2021). This was the first successful application of this approach in macaque, demonstrating plug-and-play, head-unrestrained recordings of cellular-resolution calcium dynamics from large populations of neurons simultaneously from multiple brain regions (in this study bilateral premotor cortices) during naturalistic motor behavior (Figure 1A-B). Crucially, the imaging was stable over several months, allowing the group to longitudinally track individual neurons and monitor the relationship between their activity and motor behavior over time (Figure 1C-D).
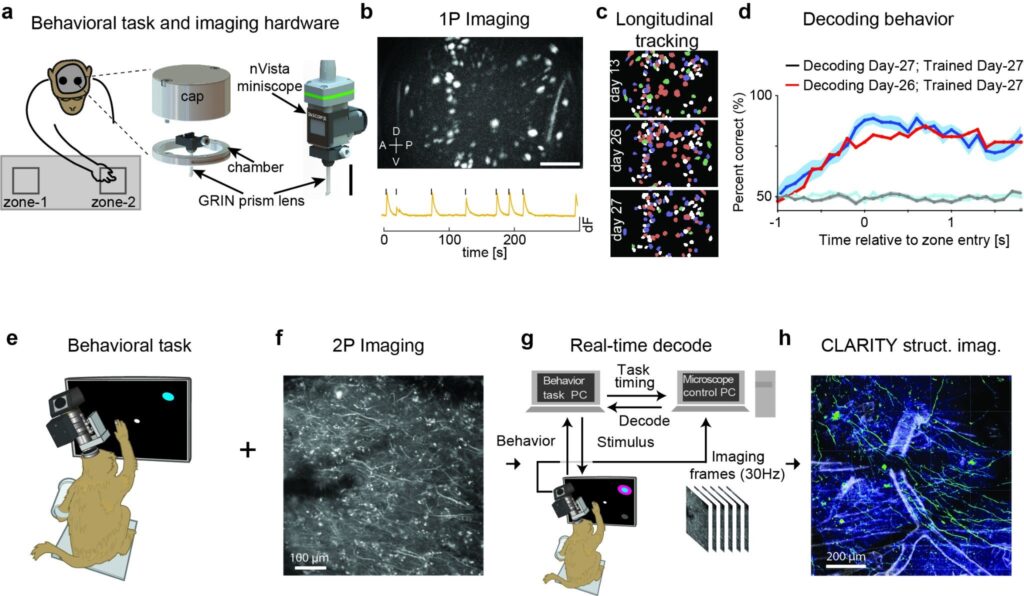
Figure 1. Two breakthrough approaches to calcium imaging and BCI in NHP. (a-d) Head-mounted microendoscopic imaging in behaving macaque monkey (a) Left: Schematic of the macaque performing the reach to reward task with two nVistaTM miniscopes (Inscopix, Inc) mounted on the head to image from bilateral PMd. Right: Zoomed in schematic of the implant hardware, including the GRIN prism lens integrated with the miniscope baseplate and the cranial chamber and cap, and the nVista miniscope docked on the baseplate for imaging. Scale bar equals 10 mm. (b) Top: Max projection image of in vivo GCaMP fluorescence over the course of a single example session. The bright colored regions in the image indicate cells that exhibited active calcium dynamics during the recording. Scale bar equals 250 mm. Bottom: Calcium activity (dF) trace of an example cell. (c) Longitudinal tracking of cells through multiple sessions. Cell maps from three different sessions spanning approximately two weeks with individual cells color-coded according to the number of sessions in which they were present and active. (d) Observed accuracy of decoding the animal’s reach direction on individual trials utilizing a model trained and tested on the same session (blue; day 27) or trained and tested on sessions 1 day apart (red; days 26 and 27). (e-h) Experimental pipeline for combining functional imaging during motor behaviors with structural imaging in macaque monkeys. (e) An NHP was trained to perform a reaching task to radially arranged targets. (f) 2P imaging was used to obtain functional signals at single-cell resolution from motor cortex (contrast-enhanced mean-intensity projection). (g) During training trials, a decoder was trained on the imaging data obtained during reaching movements. Subsequently, during test trials, this decoder was run in real time to decode (predict) the reach target from 2P imaging data. (h) Ex vivo CLARITY was performed to identify cell morphology, projection patterns and cell type (anti-GCaMP antibody labelling green, vasculature white).
A second group of researchers, led by Eric Trautmann, Daniel O’Shea, and Xulu Sun of Stanford University, demonstrated neural recordings using two-photon calcium imaging (Figure 1E-H; Trautmann, O’Shea, Sun et al. 2021). This team showed that it is possible to record signals from neurons that would otherwise be too deep in the cortex to image (without an implanted microendoscope) by imaging the apical dendrites that extend from the deep-layer cell bodies towards the brain’s surface. These apical dendrites—the tree-like fine neural processes that reach out to gather inputs from other neurons—also light up with calcium signals when the neuron they belong to fires an action potential (Beaulieu-Laroche et al. 2019), allowing the dendrites to be used as a kind of remote sensor for neurons buried deep in the cortex. By fusing two-photon functional imaging with CLARITY volumetric imaging (Chung and Deisseroth 2013), they verified that many imaged dendrites originated from layer 5 output neurons, including a putative Betz cell, a specialized class of ultra-large neurons unique to primates.
Both teams showed using their respective imaging methods, that neurons in the motor cortex were tuned to different behaviors, with each neuron firing preferentially for certain kinds of movements over others. By analyzing the imaging data, the teams were able to perform accurate offline decoding of where the monkey reached on each trial. Bollimunta, Santacruz and colleagues were able to leverage their ability to track populations of individual neurons over several weeks to investigate the stability of reach tuning over time and how that impacts the performance of their decoding algorithm. Understanding the dynamics of neural tuning and its influence on decoding performance over times scales of weeks to months will be critical toward developing so-called co-adaptive BCIs that aim to leverage neuronal adaptation (possibly via plasticity) and concomitant adaptation to the decoding algorithm to maintain or improve BCI performance over time (K. V. Shenoy and Carmena 2014). Trautmann, O’Shea, Sun and colleagues went an important step further toward demonstrating the utility of these techniques toward informing BCI development. In their study they demonstrated online, low-latency decoding capable of driving an optical BCI. By demonstrating real-time decoding capabilities, the Stanford-led team showed that these new recording methods are well-matched for implementing closed-loop experiments, where the stimulus presented to a monkey is changed based on the readout of neural activity—a mainstay experimental design of BCI studies to date. Together, these studies are particularly useful for studying fundamental neurobiology as well as developing next-generation BCIs for human patients (Sadtler et al. 2014; Golub et al. 2018; Gallego et al. 2020; Stavisky et al. 2017; Vyas et al. 2018).
While these studies are an exciting demonstration of optical-based technical capabilities in NHP, they point to considerable additional potential for future opportunities to better understand how neural circuits drive behavior and how they can be optimally leveraged to drive BCI-based therapy. We anticipate that future work will further refine the viral vector strategies available in NHP to enable cell-type specific and dense labeling of neuronal populations (Belmonte et al. 2015; Galvan et al. 2017; Inoue, Matsumoto, and Takada 2021). This will be critical for understanding the functional role of specific classes of cortical projection neurons and inhibitory interneurons and how these functions are spatially organized within the local microcircuit. Future efforts will also aim to enable optical access even deeper into the NHP brain, beyond deep layers of cortex as demonstrated here and into subcortical regions typically off-limits to large-scale population recordings. Microendoscopes that are longer yet maintain sufficient optical resolution will be critical toward this goal. Finally, both studies here involved reading out information only. Optical imaging techniques (e.g. optogenetics) allow for both recording and stimulating neurons with single-cell resolution (Marshel et al. 2019) and will be essential for studies testing the causal relationship between the functional properties of a circuit and the relevant behavior.
Together with these future developments, the breakthroughs in optical-based imaging in NHP demonstrated here will enable important new insights into the neural circuit mechanisms underlying clinically-relevant human behavior and will greatly inform our ability to develop precise and effective BCI-based therapeutics for brain injury and disease.
Articles in this post
Bollimunta, A.*, Santacruz, S.R.*, Eaton, R.W., Xu, P.S., Morrison, J.H., Moxon, J.H., Carmena, J.M., and Nassi, J.J. (2021). Head-mounted microendoscopic calcium imaging in dorsal premotor cortex of behaving rhesus macaque. Cell Reports 35 109239. https://doi.org/10.1016/j.celrep.2021.109239
Trautmann, E.M.*, O’Shea, D.J.*, Sun, X.*, Marshel, J., Crow, A., Hsueh, B., Vesuna, S.A., Cofer, L., Bohner, G., Allen, W., Kauvar, I., MacDougall, M., Ramakrishnan, C., Sahani, M., Seidemann, E., Ryu, S., Deisseroth, K., and Shenoy, K. (2021). Dendritic calcium signals in rhesus macaque motor cortex drive an optical brain-computer interface. Nature Communications https://doi.org/10.1038/s41467-021-23884-5
References
- Beaulieu-Laroche, Lou, Enrique H. S. Toloza, Norma J. Brown, and Mark T. Harnett. 2019. “Widespread and Highly Correlated Somato-Dendritic Activity in Cortical Layer 5 Neurons.” Neuron 103 (2): 235–41.e4.
- Belmonte, Juan Carlos Izpisua, Edward M. Callaway, Patricia Churchland, Sarah J. Caddick, Guoping Feng, Gregg E. Homanics, Kuo-Fen Lee, et al. 2015. “Brains, Genes, and Primates.” Neuron 86 (3): 617–31.
- Chung, Kwanghun, and Karl Deisseroth. 2013. “CLARITY for Mapping the Nervous System.” Nature Methods 10 (6): 508–13.
- Clancy, Kelly B., Aaron C. Koralek, Rui M. Costa, Daniel E. Feldman, and Jose M. Carmena. 2014. “Volitional Modulation of Optically Recorded Calcium Signals during Neuroprosthetic Learning.” Nature Neuroscience 17 (6): 807–9.
- Gallego, Juan A., Matthew G. Perich, Raeed H. Chowdhury, Sara A. Solla, and Lee E. Miller. 2020. “Long-Term Stability of Cortical Population Dynamics Underlying Consistent Behavior.” Nature Neuroscience 23 (2): 260–70.
- Galvan, Adriana, William R. Stauffer, Leah Acker, Yasmine El-Shamayleh, Ken-Ichi Inoue, Shay Ohayon, and Michael C. Schmid. 2017. “Nonhuman Primate Optogenetics: Recent Advances and Future Directions.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 37 (45): 10894–903.
- Gilja, Vikash, Paul Nuyujukian, Cindy A. Chestek, John P. Cunningham, Byron M. Yu, Joline M. Fan, Mark M. Churchland, et al. 2012. “A High-Performance Neural Prosthesis Enabled by Control Algorithm Design.” Nature Neuroscience 15 (12): 1752–57.
- Gilja, Vikash, Chethan Pandarinath, Christine H. Blabe, Paul Nuyujukian, John D. Simeral, Anish A. Sarma, Brittany L. Sorice, et al. 2015. “Clinical Translation of a High-Performance Neural Prosthesis.” Nature Medicine 21 (10): 1142–45.
- Golub, Matthew D., Patrick T. Sadtler, Emily R. Oby, Kristin M. Quick, Stephen I. Ryu, Elizabeth C. Tyler-Kabara, Aaron P. Batista, Steven M. Chase, and Byron M. Yu. 2018. “Learning by Neural Reassociation.” Nature Neuroscience 21 (4): 607–16.
- Inoue, Ken-Ichi, Masayuki Matsumoto, and Masahiko Takada. 2021. “Nonhuman Primate Optogenetics: Current Status and Future Prospects.” Advances in Experimental Medicine and Biology 1293: 345–58.
- Ju, Niansheng, Rundong Jiang, Stephen L. Macknik, Susana Martinez-Conde, and Shiming Tang. 2018. “Long-Term All-Optical Interrogation of Cortical Neurons in Awake-Behaving Nonhuman Primates.” PLoS Biology 16 (8): e2005839.
- Jun, James J., Nicholas A. Steinmetz, Joshua H. Siegle, Daniel J. Denman, Marius Bauza, Brian Barbarits, Albert K. Lee, et al. 2017. “Fully Integrated Silicon Probes for High-Density Recording of Neural Activity.” Nature 551 (7679): 232–36.
- Kao, Jonathan C., Paul Nuyujukian, Stephen I. Ryu, and Krishna V. Shenoy. 2016. “A High-Performance Neural Prosthesis Incorporating Discrete State Selection with Hidden Markov Models.” IEEE Transactions on Bio-Medical Engineering, June. https://doi.org/10.1109/TBME.2016.2582691 . Leber, Moritz, Julia Körner, Christopher F. Reiche, Ming Yin, Rajmohan Bhandari, Robert Franklin, Sandeep Negi, and Florian Solzbacher. 2019. “Advances in Penetrating Multichannel Microelectrodes Based on the Utah Array Platform.” Advances in Experimental Medicine and Biology 1101: 1–40.
- Li, Ming, Fang Liu, Hongfei Jiang, Tai Sing Lee, and Shiming Tang. 2017. “Long-Term Two-Photon Imaging in Awake Macaque Monkey.” Neuron 93 (5): 1049–57.e3.
- Macknik, Stephen L., Robert G. Alexander, Olivya Caballero, Jordi Chanovas, Kristina J. Nielsen, Nozomi Nishimura, Chris B. Schaffer, et al. 2019. “Advanced Circuit and Cellular Imaging Methods in Nonhuman Primates.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 39 (42): 8267–74.
- Marshel, J. H., Y. S. Kim, T. A. Machado, and S. Quirin. 2019. “Cortical Layer–specific Critical Dynamics Triggering Perception.” https://science.sciencemag.org/content/365/6453/eaaw5202.abstract?casa_token=jqxIMPeN5PMAAAAA:4ORY-KtIYlZlZgTtBdrTtofxuPBjb7EZv81QjO43dQxX1_xrv1dE10iqCHvjRLBKXDceuj1al2IgRA . Nuyujukian, Paul, Jose Albites Sanabria, Jad Saab, Chethan Pandarinath, Beata Jarosiewicz, Christine H. Blabe, Brian Franco, et al. 2018. “Cortical Control of a Tablet Computer by People with Paralysis.” PloS One 13 (11): e0204566.
- Nuyujukian, Paul, Joline M. Fan, Jonathan C. Kao, Stephen I. Ryu, and Krishna V. Shenoy. 2015. “A High-Performance Keyboard Neural Prosthesis Enabled by Task Optimization.” IEEE Transactions on Bio-Medical Engineering 62 (1): 21–29.
- Nuyujukian, Paul, Jonathan C. Kao, Joline M. Fan, Sergey D. Stavisky, Stephen I. Ryu, and Krishna V. Shenoy. 2014. “Performance Sustaining Intracortical Neural Prostheses.” Journal of Neural Engineering 11 (6): 066003.
- Nuyujukian, Paul, Jonathan C. Kao, Stephen I. Ryu, and Krishna V. Shenoy. 2017. “A Nonhuman Primate Brain–Computer Typing Interface.” Proceedings of the IEEE 105 (1): 66–72.
- O’Shea, Daniel J., Eric Trautmann, Chandramouli Chandrasekaran, Sergey Stavisky, Jonathan C. Kao, Maneesh Sahani, Stephen Ryu, Karl Deisseroth, and Krishna V. Shenoy. 2017. “The Need for Calcium Imaging in Nonhuman Primates: New Motor Neuroscience and Brain-Machine Interfaces.” Experimental Neurology 287 (Pt 4): 437–51.
- Pandarinath, Chethan, Paul Nuyujukian, Christine H. Blabe, Brittany L. Sorice, Jad Saab, Francis R. Willett, Leigh R. Hochberg, Krishna V. Shenoy, and Jaimie M. Henderson. 2017. “High Performance Communication by People with Paralysis Using an Intracortical Brain-Computer Interface.” eLife 6 (February). https://doi.org/10.7554/eLife.18554 . Sadtler, Patrick T., Kristin M. Quick, Matthew D. Golub, Steven M. Chase, Stephen I. Ryu, Elizabeth C. Tyler-Kabara, Byron M. Yu, and Aaron P. Batista. 2014. “Neural Constraints on Learning.” Nature 512 (7515): 423–26.
- Seidemann, Eyal, Yuzhi Chen, Yoon Bai, Spencer C. Chen, Preeti Mehta, Bridget L. Kajs, Wilson S. Geisler, and Boris V. Zemelman. 2016. “Calcium Imaging with Genetically Encoded Indicators in Behaving Primates.” eLife 5 (July). https://doi.org/10.7554/eLife.16178 . Shenoy, Krishna V., and Jose M. Carmena. 2014. “Combining Decoder Design and Neural Adaptation in Brain-Machine Interfaces.” Neuron 84 (4): 665–80.
- Shenoy, Krishna, and Byron Yu. 2021. “Chapter 39: Brain Machine Interfaces.” In Principles of Neural Science, Sixth Edition, edited by Eric R. Kandel, John D. Koester, Sarah H. Mack, and Steven A. Siegelbaum, 953–73. McGraw Hill Professional.
- Stavisky, Sergey D., Jonathan C. Kao, Stephen I. Ryu, and Krishna V. Shenoy. 2017. “Motor Cortical Visuomotor Feedback Activity Is Initially Isolated from Downstream Targets in Output-Null Neural State Space Dimensions.” Neuron 95 (1): 195–208.e9.
- Stavisky, Sergey D., Francis R. Willett, Guy H. Wilson, Brian A. Murphy, Paymon Rezaii, Donald T. Avansino, William D. Memberg, et al. 2019. “Neural Ensemble Dynamics in Dorsal Motor Cortex during Speech in People with Paralysis.” eLife 8 (December). https://doi.org/10.7554/eLife.46015 . Steinmetz, Nicholas A., Cagatay Aydin, Anna Lebedeva, Michael Okun, Marius Pachitariu, Marius Bauza, Maxime Beau, et al. 2021. “Neuropixels 2.0: A Miniaturized High-Density Probe for Stable, Long-Term Brain Recordings.” Science 372 (6539). https://doi.org/10.1126/science.abf4588 . Trautmann, Eric M., Sergey D. Stavisky, Subhaneil Lahiri, Katherine C. Ames, Matthew T. Kaufman, Daniel J. O’Shea, Saurabh Vyas, et al. 2019. “Accurate Estimation of Neural Population Dynamics without Spike Sorting.” Neuron, May. https://doi.org/10.1016/j.neuron.2019.05.003 . Vázquez-Guardado, Abraham, Yiyuan Yang, Amay J. Bandodkar, and John A. Rogers. 2020. “Recent Advances in Neurotechnologies with Broad Potential for Neuroscience Research.” Nature Neuroscience 23 (12): 1522–36.
- Vyas, Saurabh, Nir Even-Chen, Sergey D. Stavisky, Stephen I. Ryu, Paul Nuyujukian, and Krishna V. Shenoy. 2018. “Neural Population Dynamics Underlying Motor Learning Transfer.” Neuron 97 (5): 1177–86.e3.
- Willett, Francis R., Donald T. Avansino, Leigh R. Hochberg, Jaimie M. Henderson, and Krishna V. Shenoy. 2021. “High-Performance Brain-to-Text Communication via Handwriting.” Nature. https://doi.org/10.1038/s41586-021-03506-2.
